This past week I was on the special stains bench. Performing special stains was my favourite part of Histology at BCIT; from pH to incubation times and temperatures, there is a lot that goes into quality staining and I found that quite interesting.
So interesting, I took the course twice!
(Kidding, not really. I did take it twice. Not for my love of special stains, but that’s a blog for another day.)
Revisiting the workflow of Anatomical Pathology—after being grossed, embedded, and cut on a microtome, the tissue sections are stained. Most cellular structures are transparent and of similar refractive indices otherwise; tissue would be of little diagnostic value without the contrast.
Hematoxylin & Eosin (H&E) is the routine stain used in the AP lab; ‘special stains’ generally encompasses methods used to visualize features not easily seen with H&E. Nearly all of the stains I got to do were ones I had already done at BCIT, so it was worthwhile to review my old lab reports going into the week. I’ve summarized a few of them below.
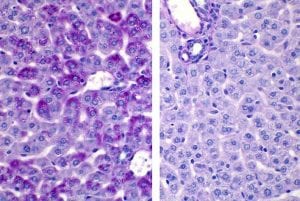
Periodic acid-Schiff +/- diastase (PAS/PASD) – PAS is a carbohydrate stain. Without going into too much detail, the method involves a reaction between Schiff’s reagent and aldehydes, which are formed from glycogen and neutral glycoproteins. Schiff’s reagent is colourless but re-establishes its chromophore function in the presence of aldehydes, staining them magenta.
Diastase is an enzyme that breaks down glycogen. So when performing PAS/PASD, two tissue sections are stained: one with diastase, one without. (Fun fact: we have alpha-amylase in our saliva, which has the same glycogen-hydrolyzing properties as diastase.) If the resulting PAS slide shows ample magenta staining and the diastase-treated section does not, you’ll know it is primarily demonstrating glycogen and not neutral glycoproteins.
Are you a BCIT News insider? Sign-up to receive the latest news on BCIT.
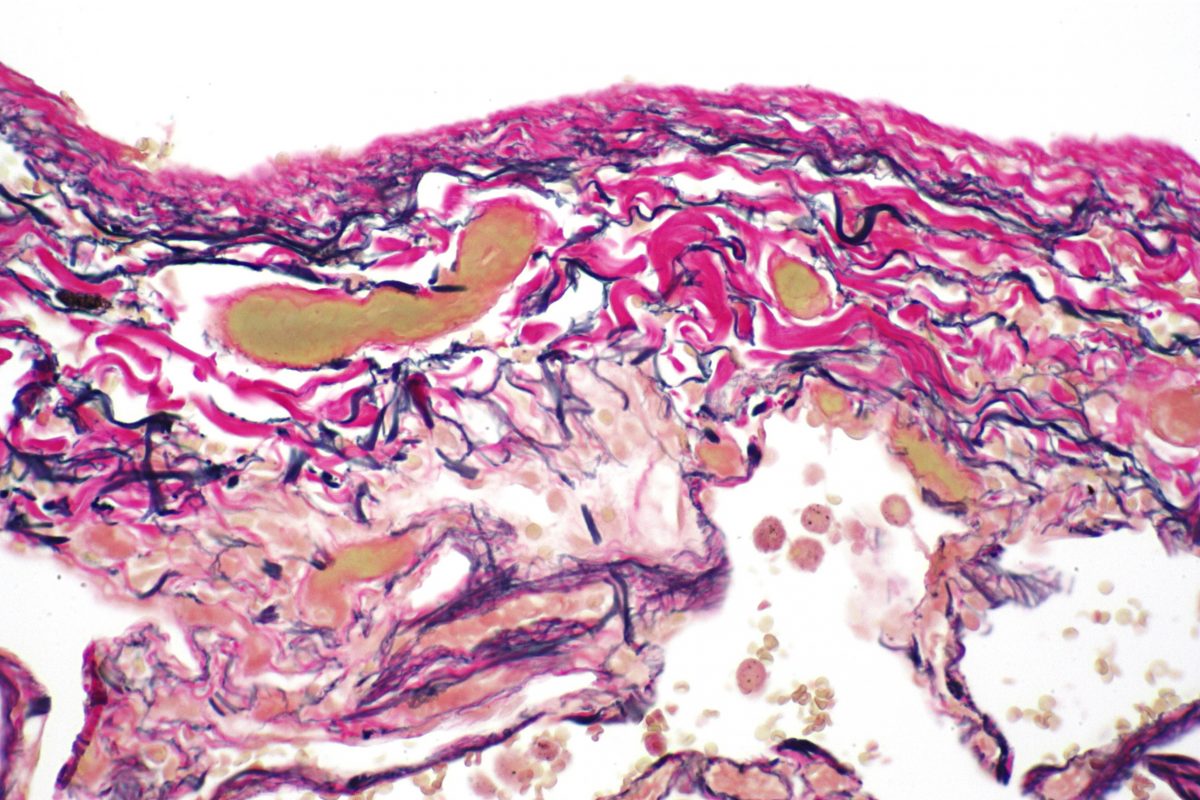
Verhoeff – Demonstrates elastin. This stain uses a regressive hematoxylin to overstain the tissue. 2% ferric chloride is then applied as a differentiating agent, removing the deep purple/black hues until only elastic fibers are staining.
Grocott’s methenamine silver (GMS) – Used to stain the cell walls of fungi, such as Pneumocystis jirovecii, black. This stain was easily the most involved. I prepared several reagents fresh (weighing out dry chemical and mixing with water), and the tissue sections are incubated in the working silver solution for up to an hour.
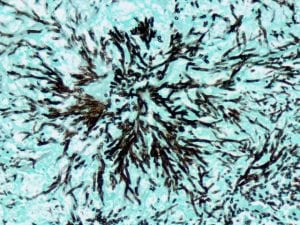
Clean glassware is always important, and even a small number of particles from past stains or tap water can affect GMS reaction(s). Prior to making up the silver solution, I rinsed the beakers, staining jars, stir rods and pipettes several times in distilled water. And you know what? I still had to do the stain four times.
Each time something different happened. When you combine the methenamine and silver nitrate solutions, it might turn cloudy but should promptly revert to a clear state. If it remains cloudy – as mine did the first time around – the solution is discarded and you start again. On another attempt, my methenamine/silver nitrate solution remained clear, but formed insoluble precipitate upon adding the borax. It was one of the more frustrating moments of my practicum thus far; I’m glad it worked out eventually.
Oil Red O – Oil Red O is the only stain of the group I did not get to perform at BCIT. It is a lipid stain and requires frozen tissue sections that are cut in a cryostat – essentially a microtome in a freezer. Lipids are soluble in alcohols and xylene, hence would be removed during routine paraffin processing.
It’s hard to believe my six-week rotation in AP is almost over! I really enjoyed the manual aspects of the department, and my trainers have been so accommodating.
Learn more about the Medical Laboratory Science full-time diploma program at BCIT.