Another three weeks in Anatomical Pathology have come and gone!
Two of the three were 1-9 PM evening shifts, which was quite an adjustment considering all my shifts prior were morning starts. I was packing sandwiches for dinner half the time; it felt weird.

The majority of each shift was spent cutting on a microtome. I’m not sure if all sites have them, but there are automated microtomes here and they are magical. I used the function for trimming blocks at 20 microns (μm) but reverted to using the manual drive wheel when taking sections to float out on the water bath.
Just to give you an idea of how thinly cut sections coming off a microtome are: while trimming – which cuts through the paraffin of a fresh block to expose its full tissue face – is carried out at 20 μm, representative sections (the tissue picked up onto a slide) are taken at ~5 μm. 1 μm is 1×10-6 metre, or one thousandth of a millimetre.
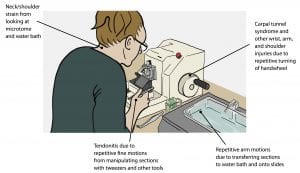
Some techs use the automated feature for both trimming and sectioning; others don’t use it at all. It comes down to personal preference—and it’s nice to have the option, given the routine tasks of the department. Embedding and/or cutting blocks involve sitting for prolonged periods of time and a lot of repetitive motion; one really has to take care of the body to lessen the risk of muscle strain or other injury.
Are you a BCIT News insider? Sign-up to receive the latest news on BCIT.
I’ll be the first to admit I have awful posture… but I also don’t want chronic neck pain before I’m 30. This diagram drives home the importance of lab ergonomics and resting the muscles every once in a while.
I wasn’t given any super tricky surgical blocks, but I did cut a fair share of brain. One of my trainers said brain and breast are the most challenging tissues to cut and I’d have to agree: fat content can contribute to less-than-ideal processing, leaving the tissue rather delicate. Once on the water bath, you may only have a few seconds to pick up the section before it explodes!
(By explode, I mean rapidly disintegrate.)
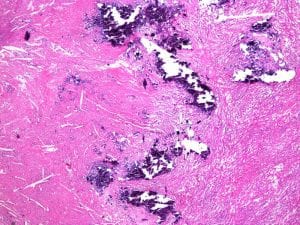
There is also potential of running into hard, unexpected deposits of calcium while trimming and/or sectioning. This often results in torn tissue sections and blade damage. A surface decalcifier – often containing acid – is used to dissolve calcium on the exposed tissue face. The block should then be rinsed in water before being chilled on ice and re-cut.
Strong acid decalcifiers (e.g. nitric acid) work rapidly, but must be carefully monitored as over decalcification can cause problems including loss of nuclear staining and tissue swelling. The block should also be re-orientated on the microtome following surface decal, as the decalcifying agent may only remove enough calcium for a few acceptable sections. Adjust distances and angles accordingly.
And don’t forget to change your blade!
Learn more about the Medical Laboratory Science full-time diploma program at BCIT.
